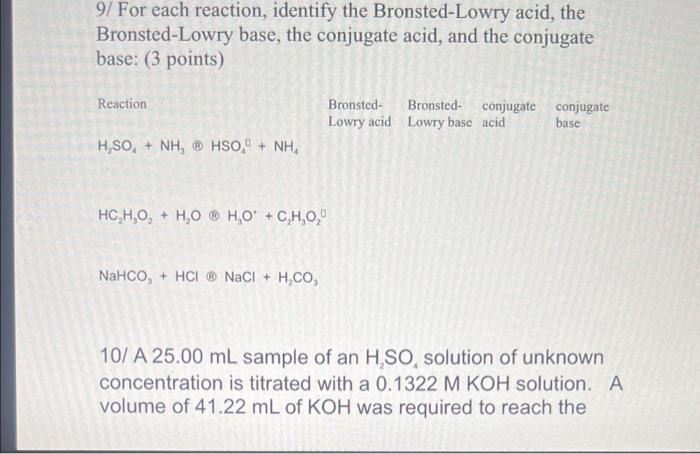
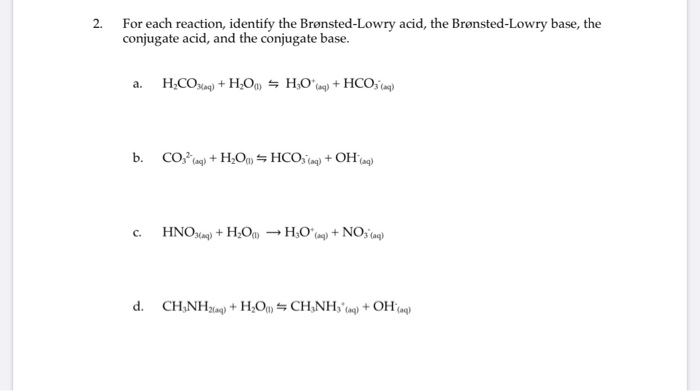
Identify the bronsted lowry acid in the following reaction – In the realm of chemistry, Bronsted-Lowry acids play a pivotal role in understanding the behavior of substances and their interactions. This comprehensive guide delves into the identification of Bronsted-Lowry acids in reactions, shedding light on their properties, significance, and applications.
Bronsted-Lowry acids are substances that donate protons (H+ ions) to other molecules in a chemical reaction. Their ability to release protons makes them crucial players in various chemical processes, including acid-base reactions and equilibrium systems.
Bronsted-Lowry Acids
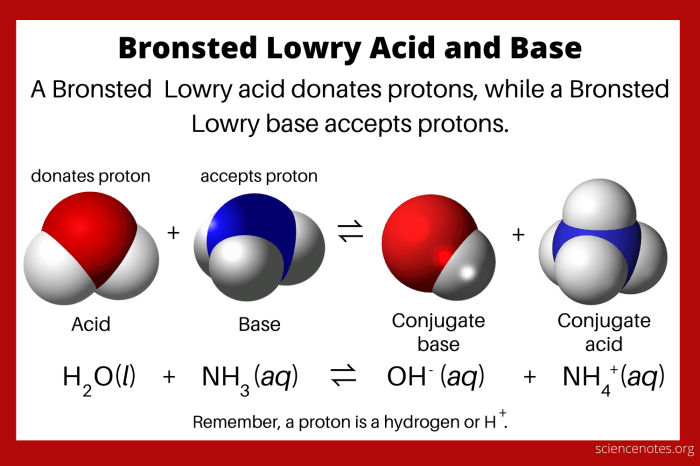
A Bronsted-Lowry acid is a substance that donates a proton (H +ion) to another substance in a chemical reaction. This proton transfer results in the formation of a conjugate base, which is the species that accepts the proton.
Examples of Bronsted-Lowry acids include:
- Hydrochloric acid (HCl)
- Sulfuric acid (H 2SO 4)
- Nitric acid (HNO 3)
- Acetic acid (CH 3COOH)
Identifying Bronsted-Lowry Acids in Reactions, Identify the bronsted lowry acid in the following reaction
To identify the Bronsted-Lowry acid in a reaction, follow these steps:
- Identify the species that donates a proton.
- Identify the species that accepts the proton.
- The species that donates the proton is the Bronsted-Lowry acid.
Examples of Bronsted-Lowry Acids
The following table lists common Bronsted-Lowry acids, their chemical formulas, and their conjugate bases:
| Acid | Chemical Formula | Conjugate Base |
|---|---|---|
| Hydrochloric acid | HCl | Cl– |
| Sulfuric acid | H2SO4 | HSO4– |
| Nitric acid | HNO3 | NO3– |
| Acetic acid | CH3COOH | CH3COO– |
Bronsted-Lowry acids have a wide range of properties and applications. For example, hydrochloric acid is used to dissolve metals, sulfuric acid is used in batteries, and nitric acid is used to produce fertilizers.
Importance of Identifying Bronsted-Lowry Acids
Identifying Bronsted-Lowry acids is important in various fields, including chemistry, biology, and medicine. In chemistry, Bronsted-Lowry acids are used to understand acid-base reactions and equilibrium processes. In biology, Bronsted-Lowry acids play a role in enzyme catalysis and protein structure. In medicine, Bronsted-Lowry acids are used to treat a variety of conditions, such as indigestion and infections.
FAQ Insights: Identify The Bronsted Lowry Acid In The Following Reaction
What are the key characteristics of Bronsted-Lowry acids?
Bronsted-Lowry acids are substances that donate protons (H+ ions) to other molecules in a chemical reaction.
How can we identify Bronsted-Lowry acids in reactions?
Identifying Bronsted-Lowry acids involves analyzing the proton transfer that occurs during a chemical reaction.
What are some common examples of Bronsted-Lowry acids?
Examples of Bronsted-Lowry acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).